Meiqi B4C boron carbide supplier!

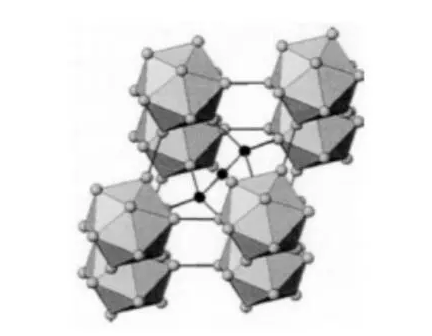
The rhombic hexahedron structure is the main crystal structure of boron carbide, and the hexahedral structure is the main lattice, the lattice constant A = 5.19A, C = 12.12A, =66°18 '.
Its unit cell contains 15 atoms (3 carbon atoms and 12 boron atoms).An icosahedron containing clusters of atoms to which covalent bonds are attached, with the three atoms attached to the diagonals of the rhombic hexahedron.
The B4C structure is generally accepted as consisting of a B11C icosahedron and a C-B-C atomic chain.There are many stable isomers (such as B13C, B12C3, B4C, etc.) between 8% and 20% of the carbon mass fraction of boron carbide due to the mutual substitution of B and C atoms in icosahedron and on the atomic chain.